Speaker
Description
This study investigates cerium migration in polymer electrolyte membrane fuel cells (PEMFCs) using a custom in-house X-ray fluorescence (XRF) spectroscopy system. Cerium serves as a crucial chemical stabilizer in fuel cells, scavenging destructive free radicals through Ce(III)/Ce(IV) redox reactions. However, prolonged operational conditions can induce complex cation migration across the active area (such as electrolyte or electrode-electrolyte interface) driven by water, potential, and concentration gradients [1,2].
The RETINA facility, developed at Politecnico di Milano, employs a customized XRF system for electrochemical devices featuring a high-power W-anode X-ray tube (40-150 kVp) and a Peltier-cooled CdZnTe detector with 400 eV resolution at 5.9 keV Fe K-shell (see Figure 1a). The system enables 1-mm resolution elemental mapping through an adjustable collimator. Quantitative elemental analysis using the fundamental parameters method is facilitated via Pymca [3].
Our investigation focused on a 140-mm commercial PEMFC, systematically examining cerium distribution in pristine and post-degradation states. Scan results demonstrated a significant transformation in cerium spatial distribution from a uniform initial profile to a pronounced concentration gradient concentrated near both electrodes after 1100h of degradation (Figure 1b and c). This non-uniform redistribution suggests active cerium migration in response to electrochemical stress, potentially accumulating at interfaces with high free radical formation and chemical instability.
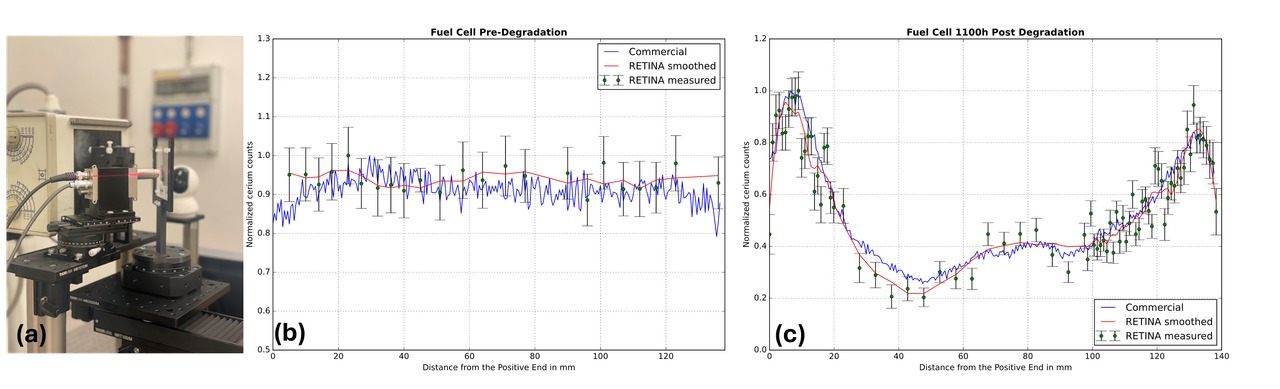
Validation was performed by comparing our results with a commercial micro-XRF Analyzer with a 500-um scan resolution, confirming RETINA’s reliability through coherent cerium concentration profiles. Comparative analysis underscores RETINA's capability to provide quantitative XRF analysis with an accessible and effective approach. The obtained concentration profiles offer valuable insights into ion transport coefficients, enabling more comprehensive modeling of mobility and diffusivity within fuel cells.
References
(1) Y.H. Lai et al., J. Electrochem. Soc. 165 F3217, 2018.
(2) A. Baker et al., J. Electrochem. Soc. 164 F1272, 2017.
(3) V.A. Sole, et al., Spectrochim. Acta B 62, 2007, 63-68
